Biomed Industries, Inc. to Present Promising NA-921 Phase 2/3 Results for Rett Syndrome Treatment at EAPS 2025
Biomed Industries, Inc. to Present Promising NA-921 Phase 2/3 Results for Rett Syndrome Treatment at EAPS Conference, October 18-20, 2025 in Lisbon, Portugal.
The Phase 2/3 results of NA-921 show strong potential as an effective, well-tolerated treatment with a favorable safety profile, promising better patient adherence and improved long-term outcomes”
SAN JOSE, CA, UNITED STATES, October 6, 2025 /EINPresswire.com/ -- - Biomed Industries, Inc. (Biomed) today announced that its Chief Executive Officer, Dr. Lloyd L. Tran, will present the topline results of Phase 2/3 clinical trials of NA-921, a novel investigational treatment for Rett syndrome at the European Academy of Paediatric Societies (EAPS), October 18-20, 2025 in Lisbon, Portugal.— Dr. Lloyd L. Tran, CEO of Biomed
The presentation, titled “PHASE 2 CLINICAL TRIALS RESULTS OF NA-921 FOR THE TREATMENT OF RETT SYNDROME", summarizes findings from a randomized, double-blind, placebo-controlled Phase 2/3 study conducted in girls and young women aged 5 to 20 years diagnosed with Rett syndrome (ClinicalTrials.gov Identifier: NCT06849973).
KEY FINDINGS:
The topline results from the study demonstrate compelling proof of safety and efficacy for NA-921, an orally administered small molecule. Biomed conducted a comparative analysis of adverse reactions observed in the clinical trials, showing a significantly improved safety and tolerability profile for NA-921.
COMPARISON WITH CURRENT TREATMENTS:
While FDA-approved treatments such as DAYBUE™ have shown clinical efficacy, they come with significant safety concerns, including high rates of severe diarrhea, vomiting, and weight loss (Treatment Management Guide for Healthcare Professionals by Acadia Pharmaceuticals, Inc. https://www.daybuehcp.com/treatment-management-guide.pdf).
A comparison of clinical trial data between NA-921 and DAYBUE revealed the following:
• Lower incidences of common side effects: Diarrhea (14% for NA-921 vs. 82% for DAYBUE) and vomiting (8% for NA-921 vs. 29% for DAYBUE).
• Improved treatment retention: Unlike the DAYBUE trial, where 35.7% of patients discontinued treatment due to adverse events, no patients withdrew from the NA-921 trial.
The details of the findings will be presented at the EAPS Conference.
These results underscore Biomed Industries’ commitment to developing safer, more effective therapies that can significantly improve patient outcomes.
“Our latest clinical data reinforce NA-921’s potential to provide a more effective and well-tolerated treatment option,” said Dr. Lloyd L. Tran, CEO of Biomed Industries, Inc. “Unlike existing therapies, which are often associated with severe gastrointestinal side effects and high discontinuation rates, NA-921 has demonstrated a remarkably low incidence of adverse events. With a favorable safety profile, NA-921 represents a promising treatment that could enhance patient adherence and long-term outcomes.”
Biomed Industries is advancing NA-921 into the next phase of clinical trials to further assess its efficacy and long-term safety.
AN UNMET NEED IN RETT SYNDROME
Rett syndrome is a rare and severe neuro-developmental disorder that primarily affects females and is often caused by mutations in the MECP2 gene on the X chromosome. Approximately 16,000 individuals in the United States and 100,000 worldwide are affected by the condition (Rett Syndrome Research Trust).
There are currently no curative treatments for Rett syndrome. Management strategies focus on alleviating symptoms and improving quality of life.
The disorder progresses through four stages, beginning with developmental delays and slowed growth between 6 and 18 months of age. As it advances, individuals experience motor impairments, loss of speech, seizures, breathing difficulties, and profound cognitive and physical disabilities, requiring lifelong care.
ABOUT BIOMED INDUSTRIES, INC.
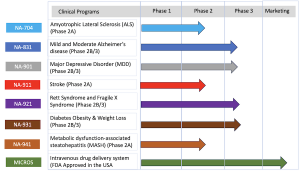
Biomed Industries, Inc. is a pioneering biopharmaceutical company dedicated to developing and commercializing novel drug therapeutics to address unmet medical needs. The company's research team has developed a new platform of drugs targeting Alzheimer’s disease, ALS, Major Depressive Disorder (MDD), Diabetes, Obesity, Metabolic dysfunction-associated Steatohepatitis (MASH), and rare diseases, including Rett Syndrome and Fragile X.
Michael Willis
Biomed Industries, Inc.
+1 800-824-5135
email us here
Visit us on social media:
LinkedIn
X
Biomed introduction video
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.